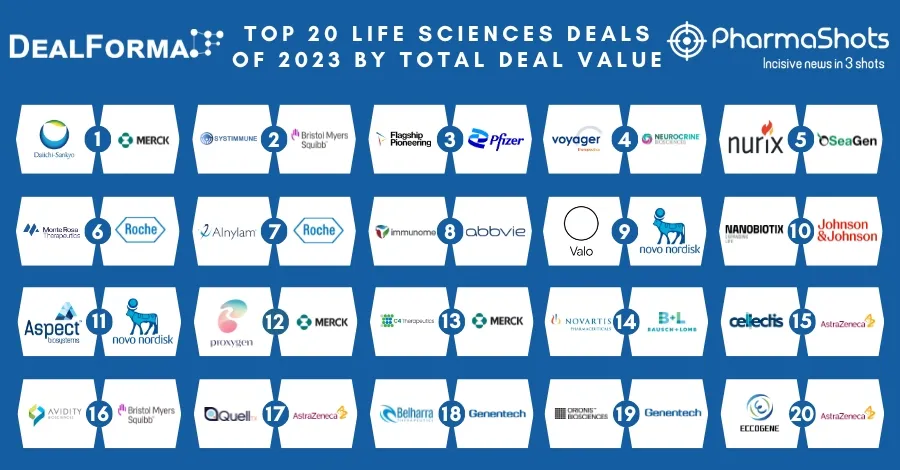
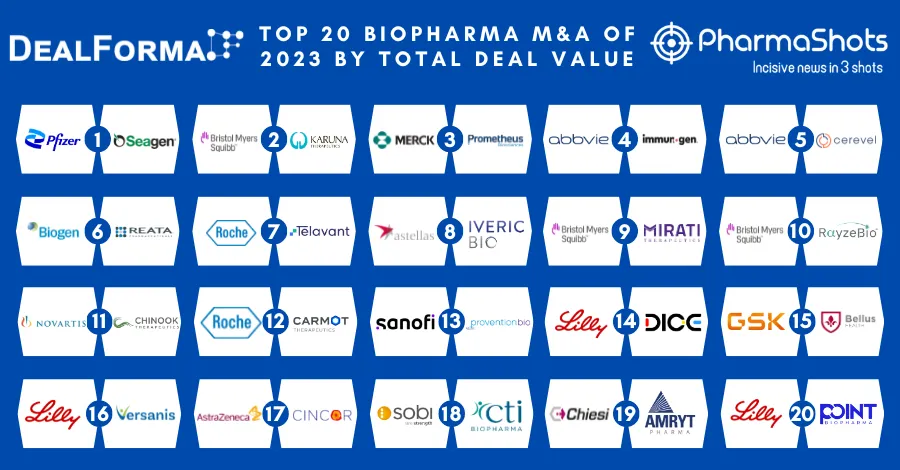
Seagen Receive the US FDA’s Accelerated Approval of Tukysa (tucatinib) for RAS Wild-Type, HER2-Positive Metastatic Colorectal Cancer
Shots:
- The US FDA has granted accelerated approval to Tukysa (tyrosine kinase inhibitor) + trastuzumab for RAS wild-type, HER2+ unresectable or metastatic colorectal cancer
- The approval was based on the P-II study (MOUNTAINEER) evaluating Tukysa (300mg, BID) + trastuzumab (8mg/kg loading dose, IV & 6mg/kg, q3w) in 84 patients, showed ORR (38%), CR (3.6%) & PR (35%), m-DoR (12.4mos.), 64% & 70% had liver or lung metastases, permanent treatment discontinuation due to AEs (6%)
- The therapy was approved in the US in combination with trastuzumab & capecitabine for HER2+ breast cancer. Merck commercializes Tukysa outside of the US, Canada & EU and will discuss (MOUNTAINEER) trial results with health authorities to accelerate Tukysa’s filing in its territories
Ref: Bussinesswire | Image: Seagen
Related News:- Seagen Receive the US FDA’s Accelerated Approval of Tukysa (tucatinib) for RAS Wild-Type, HER2-Positive Metastatic Colorectal Cancer
Click here to read the full press release

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.